
We can only measure the change it undergoes through a chemical process. That’s because we typically talk about changes, reactions or processes that actually happen in Chemistry. It’s a measurement of randomness or disorder. Thermodynamic entropy has the dimension of energy divided by temperature, which has a unit of joules per kelvin (J/K) in the International System of Units.ĭelta S is entropy. It determines that thermal energy always flows spontaneously from regions of higher temperature to regions of lower temperature, in the form of heat. Reconciling thermodynamic and state definitions of entropy. Thermodynamic entropy definition clarification. Proof: S (or entropy) is a valid state variable. In this sense, entropy is a measure of uncertainty or randomness.

Entropy is also a measure of the number of possible arrangements the atoms in a system can have. The entropy of an object is a measure of the amount of energy which is unavailable to do work.
Symbol for entropy free#
The standard Gibbs free energy of formation of a compound is the change of Gibbs free energy that accompanies the formation of 1 mole of that substance from its component elements, at their standard states (the most stable form of the element at 25 ☌ and 100 kPa). When water changes from liquid to solid, delta H is negative the water loses heat. For example, when water changes from liquid to gas, delta H is positive the water gains heat. When enthalpy is negative and delta H is less than zero, this means that a system released heat. The word enthalpy comes from the Greek en = “into” + thalpein = “to heat”. Porter proposed that H become the accepted symbol for enthalpy. Emerging Network to Reduce Orwellian Potency Yield (backronym: anonymous data store) Copyright 1988-2018, All rights reserved.Įxplanation: In 1922, Alfred W. What is the abbreviation for entropy?Īcronym.
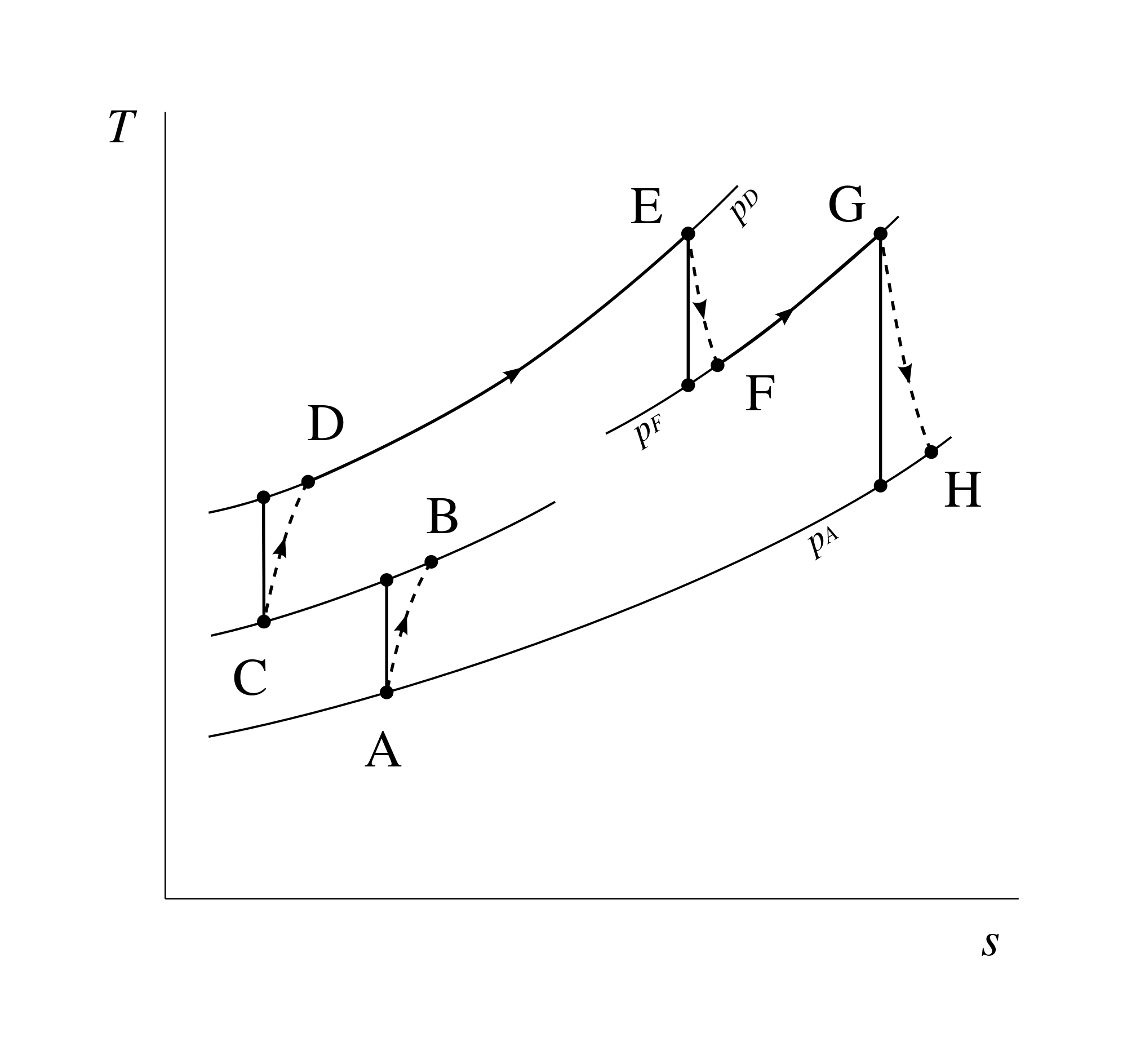
*If the work done by the *system is greater than the heat absorbed by the system, E is negative. *For an endothermic process, H is negative. *The thermodynamic symbol for entropy is S. Is the thermodynamic symbol for entropy is S? His 1824 research paper was studied by Clausius over many years.Įntropy Definition In equations, entropy is usually denoted by the letter S and has units of joules per kelvin (J⋅K−1) or kg⋅m2⋅s−2⋅K−1. 2 What is the abbreviation for entropy?Įxplanation: It is generally believed that Rudolf Clausius chose the symbol “S” to denote entropy in honour of the French physicist Nicolas Sadi-Carnot.


 0 kommentar(er)
0 kommentar(er)
